How much protein should you eat per day? While the focus in recent decades has been on fats and carbohydrates, today more and more research shows that getting enough protein in your diet is very important. They even remembered that the word “protein” comes from the Greek protos, which means “first”.
Apart from water, bones and fat, the human body consists of protein. The most protein is in the muscles, but that’s not all: the heart, blood vessels, ligaments, tendons, skin and hair are also protein. And also hormones, enzymes, cells of the immune system. And all this must be updated. Different body tissues renew themselves at different rates. Plasma proteins - within several hours. Muscle proteins - in a few days. Renewal of connective tissue can take months and even years.
Muscle growth and maintenance depend on the protein in your diet. Recovery from illness and stress also depends on adequate amounts of protein. The effectiveness of a diet for weight loss also requires a lot of protein in food. So protein is important for both health and figure. But how much is needed? There is no clear answer. The amount of protein depends on a person’s physical activity, health status, age and nutrition.
The RDA (US National Dietary Guidelines for Health) recommends 0.8 grams per kilogram of body weight. This is the minimum for people who do not exercise or diet (). More recent studies say that the same non-athlete needs more protein - 1-1.2 grams per kilogram of body weight.
The new norm has not yet become official, although since the publication of the current protein rda, many studies have shown some benefits from increasing protein in food: maintaining muscle mass even in old age (prevention of osteoporosis), improving nutritional quality, weight control and improving body composition.
In 2013, the second The Protein Summit took place (the first was held in 2007), the results of which were published in the American Journal of Clinical Nutrition. The purpose of the summit is to discuss the role of protein in human health and explore the misconception that Americans eat too much protein, above RDA guidelines. More than 60 nutrition researchers, health experts and nutritionists attended the summit.
Based on research presented at the summit, increasing your protein intake by twice the recommended amount is not only safe, but also beneficial. This works out to be approximately 15%-35% protein as a share of total daily calories, but may be higher or lower than this range depending on age, gender and activity level.
The American College of Sports Medicine ACSM recommends consuming 1.5-2 grams of protein per kg of body weight per day for strength training, and 1.2-1.4 grams per day per kg of body weight for endurance training. And he calls 2 grams the extreme limit, above which protein is not harmful, but no longer has additional benefits.
The National Strength and Conditioning Association NSCA also recommends 1.5-2 grams per kilogram of body weight per day for exercisers.
Some studies suggest that protein intake in athletes can be increased to 3 g/kg per day, this is not harmful and may have additional benefits - although not dramatic.
The International Society of Sports Nutrition recommends 1.4-2.0 g/kg body weight for physically active people, and says that this is not only safe, but also beneficial for the body's adaptation to stress (,).
The American Dietetic Association also supports high protein intakes for active people () in the range of 1.2-1.7 g/kg body weight.
Lyle MacDonald in his book The Protein book, based on various studies of protein norms for athletes, gives the following figures.
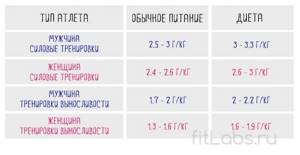
How much protein do you have on your diet?
All protein recommendations are given for weight maintenance, but not for a calorie deficit diet. It is assumed that in states of energy deficiency, additional protein helps maintain muscles. A systematic review of studies on protein requirements for weight-training adults who are not overweight found that the norm is 2.3 to 3.1 grams per kilogram of lean body mass.
It's difficult to say with 100% certainty that eating more protein will help you maintain more muscle mass while dieting. But it is safe to say that 2.5-3 g/kg dry body mass will not be harmful, despite the fact that this is higher than traditional recommendations. Some studies have shown that weight does not increase on a high-protein diet, despite an intentional caloric excess (, , , ). Finally, a higher protein diet prolongs feelings of fullness and has a positive effect on mood, in contrast to a diet with lower protein intake ().
Meat and milk proteins
Meat, milk and products derived from them contain proteins necessary for the body, which are favorably balanced and well digestible. Proteins of muscle tissue of animal meat are complete, according to
The balance of amino acids between beef, lamb and pork differs little from each other. Proteins in connective tissue and cartilage are deficient. In the human and animal body, muscle proteins perform a contractile function, while connective tissue and cartilage proteins perform a structural function. The functions of all these types of proteins are based on their fibrillar nature.
The protein content in meat products ranges from 11 to 22%. The main muscle proteins are myosin
and
actin,
the molecular function of which is to provide a mechanism for muscle contraction and relaxation with the participation of ATP. Myosin makes up 55% of muscle protein by mass and is a hexamer with a molecular weight of 460 kDa. The hexamer includes a fibrillar part (two intertwined α-helices with a molecular weight of 200 kDa, ending in globular “heads”), heavy chains and two pairs of light chains (molecular weight 15-27 kDa). Myosin has ATP-hydrolyzing activity and the ability to bind to insoluble F-actin. Actin is a monomeric globular protein with a molecular weight of 43 kDa (G-actin), which accounts for 25% of the total muscle protein mass. In the presence of magnesium, G-actin undergoes non-covalent polymerization to form a double helical chain, called F-actin. Muscle contraction involves the repeated attachment and detachment of the globular myosin head from the F-actin filament. ATP hydrolysis initiates a cycle of association and dissociation of actin and myosin in five reactions of this process (Fig. 2.16).
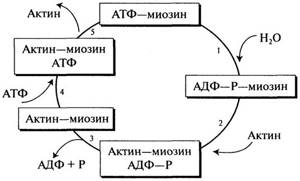
The essence of the muscle relaxation process is the separation of the myosin (ATP) head from F-actin.
Muscle cells contain the globular hydrogen soluble chromoprotein myoglobin,
having a heme-cyclic tetrapyrrole as a prosthetic group, the presence of which explains the red color of this protein. Tetrapyrroles consist of four pyrrole molecules linked by four α-methylene bridges to form a planar ring structure. In the center of the flat ring there is one iron atom in the ferro state (Fe2+), the oxidation of which leads to the loss of biological activity of myoglobin.
The biological function of myoglobin is not to transport oxygen, like hemoglobin, but to store it. Under conditions of oxygen starvation (for example, during physical exercise), oxygen is released from the complex with myoglobin and enters the mitochondria of muscle cells, where ATP synthesis occurs (oxidative phosphorylation).
Using myoglobin as an example, the relationship between the function of a globular protein and its structure has been well studied. Myoglobin consists of one polypeptide chain with a molecular weight of 17 kDa, including 153 amino acid residues. Approximately 75% of the residues form eight right-handed α-helices arranged in a compact spherical molecule. In places where the polypeptide chain bends, there are proline, serine, and threonine residues that are not capable of forming an α-helix. There are polar residues on the surface of the molecule, and nonpolar ones inside, with the exception of two histidine residues that take part in the binding of oxygen. The addition of oxygen to myoglobin is accompanied by a displacement of the iron atom, and with it histidine, and covalently bound other amino acid residues in the direction of the plane of the heme ring. As a result, this region of the protein molecule takes on a new conformation.
Myoglobin not bound to oxygen is called deoxymyoglobin (Mb), oxygenated Mb is called oxymyoglobin (MbO2). The color of meat products depends on the myoglobin content, the state of the meat and the protein part of the macroglobule. Oxidation of Fe2+ in myoglobin to Fe3+ leads to a change in pigment color from bright red to dark brown, since the resulting metmyoglobin (MetMb) loses its ability to bind molecular oxygen. Thermal denaturation of globin also leads to the loss of the heme pigment's ability to bind oxygen and deteriorates the color of products.
Myoglobin oxygen can be replaced by ligands such as nitric oxide, carbon monoxide, etc., therefore this property of the protein
muscle tissue of meat is used to obtain intense coloring of meat products. Nitrite (NO), used for this purpose, reacts with myoglobin to form nitrosomyoglobin, which, when heated, turns into a stable red pigment, nitrosomyochromogen:
Myoglobin and its derivatives have different spectral characteristics; therefore, spectrophotometric analysis methods are used to identify them when assessing the quality of meat.
The most abundant protein in the animal kingdom is collagen.
main macromolecule of skin, tendons, blood vessels, bones, cornea and cartilage. It provides extracellular structure in animal connective tissue, existing in no less than five different types. The main feature of collagen molecules is a three-helical structure, each a-chain (subunit) of which is a left-handed helix. The helix has three amino acid residues per turn. Three left a-helices twist into right-handed superhelices, which in turn combine into fibrils.
Another characteristic feature of the collagen molecule is the presence of glycine a-chain as the third residue of the triple helix. The repeating structure can be represented as 1li-X-Y, where X, Y are other amino acids. About 100 X- and 100 Y-positions in collagen are occupied by proline and 4-hydroxyproline, respectively (see Amino acids and some of their functions in the body). Some X positions contain 3-hydroxyproline, and some Y positions contain 5-hydroxylysine. “Hard” amino acid residues increase the stability of the triple helix. The amount of hydroxyproline determines the degree of collagen digestibility when assessing the quality of meat.
Collagen is an extracellular protein, but it is synthesized inside the cell as precursor molecules, passing through the endoplasmic reticulum and the Golgi complex. As a result of the process of post-translational modification, the collagen triple helix is stabilized between intrachain disulfide bonds, o-glycosidic bonds between saccharide and hydroxylysine residues, and cross-linking of chains and helical fibril molecules through Schiff bases (see Chapter 3) and aldol condensation.
The protein elastin, which is similar in properties to collagen, is found in the elastic fibrils of connective tissue .
contained in ligaments and walls of blood vessels. This protein is rich in glycine, alanine and lysine, but poor in proline. A distinctive feature of elastin
is the presence of unusual cross-links in its structure. Aldehyde groups resulting from the oxidation of the amino groups of the side chains of lysine and oxylysine residues interact with the amino group of lysine through aldol condensation, dehydration and oxidation reactions, forming desmosine and isodesmosine. All four amino groups and carboxyl groups are involved in the formation of peptide bonds.
Meat containing a lot of connective tissue remains tough even after heat treatment; the digestibility of collagen and elastin in it is very low. However, if it is necessary to enhance intestinal motor function, it is advisable to use foods rich in connective tissue. Gentle diets use gelatin, a product of incomplete hydrolysis of collagen. Gelatin is incomplete in its amino acid composition, but jelly-like products made from it are digested without straining the secretion of the digestive organs.
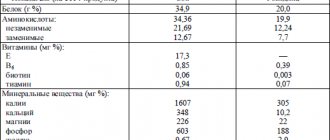
Milk is a heterogeneous system in which emulsified fat globules and colloidal casein micelles act as the dispersed phase,
and as a dispersion medium - a solution of proteins, lactose, salts and vitamins. The total protein content of milk ranges from 2.9 to 3.5%. Among them, two main groups are distinguished: breech proteins and whey proteins (Table 2.12). Milk contains more than 20 enzymes (xanthine oxidase, peroxidase, catalase, lipase, cholinesterase,
Table 2.12. Composition and molecular characteristics of milk protein components
| Components | Content | Molecular weight, kD | IET | |
| in % of total proteins | in g/l | |||
| Casein: | 78-85 | … | ||
| αs1-casein | } 43-54 | 12-15 | 23,0 | 4,4-4,8 |
| αs2-casein | 3-4 | 25,0 | — | |
| β-casein | 25-35 | 9-11 | 24,0 | 4,8-5,1 |
| χ-casein | 8-15 | 2-4 | 19,0 | 5,4-5,8 |
| Whey proteins: | 15-25 | 6-8 | ||
| β-lactoglobulin | 7-12 | 3,6 | 18,3 | 5,1 |
| α-lactalbumin | 2-5 | 1,7 | 14,2 | 4,2-4,5 |
| immunoglobulins | 1,5-2,5 | 0,6 | 150-1000 | 5,5-8,3 |
| serum albumin | 0,7-1,3 | 0,4 | 69,0 | 4,7-4,9 |
α-amylase, lysozyme, protease, etc.), as well as hormones (prolactin, oxytocin, corticosteroids, thyroxine, triiodothyronine, etc.) and proteins in the membranes of fat globules.
The main proteins of milk are breech proteins, which are easily digested and are sources of essential amino acids, calcium, phosphorus and a number of physiologically active peptides. Thus, when chymosin acts on k-casein in the stomach, glyco- and phosphopeptides are released, which regulate the secretion of gastric juice, model the physicochemical properties of proteins (solubility, viscosity, charge, denaturation), protect against proteolysis and affect membrane permeability cells. Whey proteins also have important physiological functions. Immunoglobulins perform a protective function, lactoferrin and lysozyme (enzyme) are carriers of antibacterial properties, and lactoferrin and β-lactoglobulin perform a transport role, transferring micro- and macroelements, vitamins and lipids to the intestine. α-Lactalbumin is necessary for the synthesis of lactose in milk from UDP-galactose and glucose.
For most components of casein, α-lactalbumin, β-lactoglobulin and components of the protease-peptone fraction, the primary and some fragments of the secondary, tertiary and quaternary structure have been deciphered. Thus, casein molecules have a small amount of α-helices - 1-6% (while, for example, α-lactalbumin consists of 26% α-helix, β-conformation) and only the rest of the protein is a disordered structural organization. In the formation of the quaternary structure of casein, a large role is given to hydrophobic interactions and calcium phosphate bridges and a smaller role to electrostatic and hydrogen bonds. Calcium phosphate bridges are the basis of the caseinate calcium phosphate complex, in the form of which casein is contained in milk:
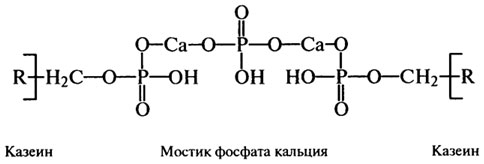
Calcium in the complex acts as a structure former, creating a bridge between the serine phosphate groups of two casein molecules, and phosphoric acid residues enhance the acidic properties
protein, due to the presence of β- and γ-carboxyl groups of aspartic and glutamic acids in polypeptides. Casein from milk precipitates at a pH of 4.6-4.7, when its molecules become equal in positive and negative charges. Precipitated casein is practically insoluble in water, but dissolves in a slightly alkaline medium and solutions of alkali metal salts and mineral acids. Insoluble casein has the ability to bind water in fairly large quantities (more than 2 g per 1 g of protein), which is very important for the stability of protein particles in raw, pasteurized or sterilized milk. The hydrophilic properties of casein are enhanced by its interaction with p-lactoglobulin, which is observed during the heat treatment of milk, and the structural and mechanical properties of clots formed during acid coagulation or the production of cheese mass during the ripening of cheeses depend on them.
Industrial breech milk is produced from skim milk by the action of acids, fermented whey, and the introduction of calcium salts, chymosin or other enzymes. Depending on the production method, sodium caseinate, calcium caseinate, acid casein, rennet casein and coprecipitate with different functional properties are distinguished. To regulate the latter, incomplete enzymatic hydrolysis or mixing with plant proteins and their co-drying are often used. In the production of new forms of protein foods (analogues of meat and fish products), the gelation of casein, its interaction with non-protein substances, the formation of stable emulsions and the phenomenon of syneresis are of great importance.
Milk proteins are characterized by high biological value; they contain excess amounts of lysine and tryptophan with a simultaneous lack of sulfur-containing amino acids (see Nutritional and biological value of proteins). Whey proteins contain essential amino acids in significantly higher quantities than casein, including lysine, threonine, tryptophan, methionine and cysteine.
Whey proteins account for 0.5-0.8% of the total amount of proteins in milk. β-Lactoglobulin is resistant to the action of pepsin in the acidic environment of the stomach, therefore it is broken down only in the intestine by trypsin and chymotrypsin. During the pasteurization of milk, the protein is denatured, forming complexes with κ-casein, and is precipitated along with it during acid and rennet coagulation. The pliability of this complex to the action of rennet is reduced. α-Lactalbumin does not precipitate at the isoelectric point (pH 4.6), does not coagulate under the influence of rennet, and is thermostable due to the large number of disulfide bonds. Immunoglobulins are glycoproteins by chemical nature. They perform their function
causing agglutination of microorganisms and other foreign cells. Three main groups of immunoglobulins have been identified: G, A and M. Among them there are monomers and polymers consisting of heavy and light polypeptide chains with a molecular weight of 50 kDa and 25 kDa, respectively.
There are two main types of whey: sweet, formed during the production of cheese, and sour, obtained during the precipitation of cottage cheese and caseins. To use whey as an additive in the baking and confectionery industry, and for the production of infant formula, it is concentrated by drying, ultrafiltration, electrodialysis, and protein precipitation in the form of complexes with polyelectrolytes. To obtain isolates, concentrates and coprecipitates, thermal denaturation is used, followed by precipitation of the protein in IET (pH 4.5-4.6) and complexation with anionic polysaccharides (CMC, alginates and pectins). These methods make it possible to isolate up to 70-90% of complete whey protein and vary its functional properties.
79 :: 80 :: 81 :: 82 :: 83 :: 84 :: 85 :: 86 :: Contents
86 :: 87 :: 88 :: 89 :: 90 :: 91 :: Contents
2.7. NEW FORMS OF PROTEIN FOOD. PROBLEM OF PROTEIN ENRICHMENT WITH LIMITING AMINO ACIDS
The main direction of scientific and technological progress in the field of food production in the last three decades has been the intensification of food preparation processes while simultaneously imparting to it a set of properties that reflect the requirements of the science of healthy nutrition. New food production includes technologies for producing protein products as a priority. These technologies are based on fundamental and applied knowledge in the field of food, physical, biological, bioorganic chemistry, genetics, molecular biology, biophysics and a number of technical disciplines. The objective reasons for the creation of fundamentally new technologies for obtaining protein components of food are the following: population growth, people’s awareness that the planet’s resources are not limitless, the need to produce food products with a composition that corresponds to the modern way of life, and the possibility of using theoretical knowledge accumulated by man for applied purposes. A distinctive feature of protein product production technologies is the possibility of targeted use of individual protein fractions and complex processing of raw materials with
simultaneous receipt of other useful food ingredients (starch, oil, pectin, phosphatides, etc.).
New forms of protein food are food products obtained from various protein fractions of food raw materials using scientifically based processing methods and having a certain chemical composition, structure and properties, including biological value.
An objective quantitative assessment of the creation and development of the industry for the production of plant protein products (fractions) is the availability of agricultural raw materials, high-performance equipment (extractors, separators, centrifuges, dryers, etc.) and competitive technologies. Potential raw material sources include: legumes (soybeans, peas, lentils, lupine, beans, chickpeas); grain and cereal crops (wheat, triticale, rye, oats, barley, corn) and by-products of their processing (bran, chaff, flour, germ); oilseeds (sunflower, flax, rapeseed, sesame); pseudocereals (amaranth); vegetables and melons (potatoes, pumpkin); vegetative mass of plants (alfalfa, clover, lupine, sugar beet, green tobacco); processed fruits and berries (apricot pits, plums, cherries, dogwoods, grapes, etc.); pine and other types of nuts. No less important factors determining the choice of raw materials are: the amount and composition of protein, biological value, the ability to remove antinutrients, functional properties, storage ability, the possibility of deep fractionation to obtain both main (protein) and nutritional by-products (fat). , starch) or therapeutic and prophylactic (pectin, sorbitol, xylitol, lecithin, anthocyanins, vitamins, glucose-fructose syrups, etc.) value. Food protein production facilities are built close to biochemical or feed factories in order to obtain a number of additional ingredients (yeast, enzyme preparations, dry pulp, etc.) or specialized workshops are organized at existing enterprises.
Traditional sources for the production of protein products are soybeans and wheat. Soy protein products are divided into three groups, differing in protein content: flour, concentrates, isolates. Based on these types of protein products, the production and marketing of textured flour, concentrates and isolates is organized. Modified and special protein products are produced. Soybean meal and grits are produced in milling equipment by grinding defatted or non-defatted seeds to a specific particle size and then sifting them. Flour and cereals contain 40-54% (N x 6.25) protein of the total mass of the product. Different types of flour (cereals) differ in fat content,
particle size and degree of heat treatment. KRA, KDB, the activity of the enzymes lipoxygenase, urease and protease inhibitors depend on the intensity of thermal exposure. Soy protein concentrates are made from hulled and defatted soybeans (white petals) by removing water-soluble non-protein components (oligosaccharides, enzymes, minerals). Concentrates contain 65-70% protein on a dry matter basis (N x 6.25). Soy protein isolates are the most purified form of protein products, as they contain at least 90% protein on a dry matter basis. The protein is extracted from the crushed white petal with a slightly alkaline solution (pH 8-11), followed by precipitation at the isoelectric point (4.2-4.5) and separation in the form of a curd mass from the oligosaccharides. The protein mass is washed, neutralized to pH 6.8 and dried.
The purpose of textured protein products is to impart a fibrous or multi-layer (lumpy) structure to food products. After hydration, such protein products resemble meat, poultry or seafood in appearance and structure, while acting as analogues of traditional food products. The multilayer meat-like structure of soy protein products can be formed using thermoplastic extrusion. The main stages of the process include: dosing of raw materials → conditioning (humidification, heating) → cooking process → laminar flow (orientation of protein molecules) → fiber formation → cutting the product into pieces → drying. Extrusion is based on the process of protein restructuring, which consists in the fact that under the influence of temperature, moisture and mechanical action, its macromolecules form a viscoplastic mass, aligned in the direction of shear, with the formation of new cross-links. As a result, a multi-layered three-dimensional chewing structure is formed, suitable for use as fillers or analogues.
Specialty soy products include soy sauce, tofu (bean curd), soy milk, miso (soy paste) and other types. Modified proteins (partially or completely hydrolyzed) are obtained from protein products using proteolytic enzyme preparations (pepsin, papain, bromelain) or acid hydrolysis. Such proteins are used as functional and flavoring food additives.
Dry wheat gluten is obtained from wheat or wheat flour by water extraction of non-protein and soluble protein components. Since gluten is a perishable product, drying occupies an important place in the technological process of gluten production. Firstly, the moisture content of the finished product
the product content should not exceed 10%, and secondly, the gluten should be native, or “vital”. The first condition is necessary for successful storage, and the second is necessary to ensure the widespread use of gluten as a technofunctional ingredient. Gluten contains at least 75-80% protein (N x 5.7), fat - 0.5-1.5%, fiber - 1.5%, ash content - 0.8-1.2%.
Despite the fact that a huge number of technologies have been developed and implemented in the food protein industry, there are promising areas that consist in obtaining protein products of increased nutritional and biological value from non-traditional raw materials, fractionating proteins into components with different molecular weights (for example, 7S and 11S proteins soybeans), physicochemical, functional and pharmacological characteristics and the development on their basis of a new generation of protein supplements (composites) with multifunctional properties and multi-purpose applications.
Plant protein of grains and other crops in the total mass is inferior to animal protein in the content of essential amino acids (lysine, threonine and tryptophan). Therefore, today, throughout the world, special nutrition programs are being widely developed and implemented, providing for the use of plant proteins or limiting amino acids for adults, schoolchildren and children. The optimal balance of essential nutritional factors is ensured by the correct selection and combination of different types of proteins (mutual enrichment effect). Supplementing food, for example, with soybeans, is an excellent method of replenishing the deficiencies of lysine in wheat, corn and rice. Due to the correct selection of components in mixtures of bread, legumes and oilseeds can significantly increase the EBC, the standard for which is taken to be casein (2.5).Mixtures of bread crops with soy products are complementary in amino acid composition already at a ratio of 50:50, but 30:70 is considered ideal.
Depending on the ratio of protein components, the effects of true and simple enrichment are distinguished. The effect of true enrichment is observed if the score for each essential amino acid in the protein of the product being created is at least 1.0, and simple - if the values of the amino acid score of the composition, although less than 1.0, are higher than the values of this indicator for the proteins of each product separately. The balance of the amino acid composition in protein products has a positive effect on their digestibility. If the digestibility of plant proteins compared to casein is 60-80%, then the digestibility of proteins contained in concentrated protein products with a large number of essential amino acids is 80-100%. Thus, the digestibility of soy protein by an adult
concentrates and isolates, like milk proteins, are in the range of 91-96%, dry wheat gluten - 91%, and protein flour from wheat bran - 94%. This figure can be increased to 97-99% by mixing them with limiting amino acids. For example, adding 0.5-1.5% methionine to soy protein products brings them closer in nutritional value to ideal protein, and adding tryptophan to food causes the formation of antibodies in the human body and increases its immunity. In table Figure 2.13 shows the effect of amino acid supplements on the quality of grain feed, assessed by EBC.
Limiting amino acid additives have long been used in the production of food and feed, the production of which is a large-scale specialized industry in the world. More than 98% of production (according to FAO) comes from methionine, lysine and tryptophan. The main methods for obtaining amino acids are microbiological (lysine, threonine, valine) and chemical (methionine, tryptophan, phenylalanine) synthesis methods, however, some of the deficient amino acids can be obtained using enzymatic methods (methionine), extraction (cystine, tyrosine) and genetic engineering (lysine , threonine).
The consumption of amino acids in food requires careful monitoring by doctors and nutritionists, since it requires special methods of consumption and administration methods. Limiting amino acids, being in food and not participating in cavity digestion, will either quickly enter the circulatory system or remain in the intestine, where, under the influence of microflora, they will become the object of the formation of toxic products. The difference in the time of entry into the blood of free amino acids and amino acids formed during the digestion of food proteins will contribute to the occurrence of negative enzymatic transformations of deamination, decarboxylation, etc. Free amino acids, not taking part in the synthesis of body proteins, can become a source of toxic biogenic amines and ammonium salts. The most toxic products are
Table 2.13. Effect of amino acid supplements on the BEC of grain crops
| Grain crop | Amino acid | KEB | |
| without additive | additive | ||
| Wheat | L-lysine (0.2%) | 0,7 | 1,6 |
| L-lysine (0.4%) + DL-threonine (0.3%) | 0,7 | 2,7 | |
| Rice | L-lysine (0.02%) + DL-threonine (0.2%) | 1,5 | 2,6 |
| Corn | DL-lysine (0.4%) + L-tryptophan (0.07%) | 0,9 | 2,6 |
deamination of tryptophan, tyrosine, histidine. Thus, histamine and serotonin, formed by the decarboxylation of histidine and tryptophan, respectively, are allergy-causing substances.
Consumption of valuable plant proteins in food generally has a positive effect on people's health. By supplying the body with essential amino acids, protein products are a source of dietary fiber that can form structural complexes with the therapeutic and physiological function of influencing intestinal motility and regulating cholesterol levels in the blood. Plant proteins reduce the level of serum lipids in patients with hyperlipidemic conditions (atherosclerosis, hypertension, diabetes mellitus, cholelithiasis, endocrine disorders, etc.), and therefore interest in replacing animal proteins with plant proteins has especially increased in recent years. Thus, replacing meat and dairy products with soy protein isolates in the diet of patients with high levels of lipoproteins and cholesterol in the blood reduces the level of total cholesterol and cholesterol with LMP.
86 :: 87 :: 88 :: 89 :: 90 :: 91 :: Contents
91 :: 92 :: 93 :: 94 :: 95 :: 96 :: 97 :: 98 :: 99 :: 100 :: 101 :: Contents
Adviсe
- Physically active people should eat more protein than sedentary people.
- On a diet, the amount of protein can be increased to protect muscles. During times of dietary energy deficiency, the body increases oxidation of the amino acid leucine (converting it into glucose), which requires a higher dose of leucine in the diet to restore and maintain nitrogen balance (, ,).
- If you have a lot of excess weight (from 25-30% body fat and above), then the protein norm should be calculated by weight of dry body mass. To do this, you need to find out your fat percentage, subtract it from your total weight, and do all calculations based on the resulting “fat-free” weight.
- A common beginner mistake is to confuse the weight of pure protein with the weight of a product containing protein. When we recommend eating 150 grams of protein per day, we mean pure protein, not 150 grams of chicken breast or cottage cheese. One hundred grams of cooked chicken breast contains about 30 grams of pure protein.
- “Eat more protein” does not mean “eat more meat.” Meat is one of the sources of protein. Eggs, cottage cheese, fish, seafood, legumes are also its sources. Any protein is taken into account in total - and from plants too.
- It is important to remember that in food products protein is often found in combination with large amounts of carbohydrates (legumes and cereals) or fats (low-fat milk, nuts, fatty meats and fish, cheeses, sausages, ham). The goal is to try to choose lean protein - white poultry, lean fish, lean cuts of beef, low-fat cottage cheese.
Best Source of Protein
There is still fierce debate about this. Some argue that it is imperative to consume animal products and provide figures as proof of how much protein is in beef. A significant part of bodybuilders eat specially selected meat and fish products. Others, on the contrary, believe that meat can be eaten only once or twice a week, and even then in small portions. Let's try to find out today which product is most suitable for replenishing protein reserves in the body.

Beneficial features
No other product provides your body with as much healthy protein as the protein in 100 g of beef. This is the main supplier in our modern realities. When cooking young meat, no more than 2% of protein is lost. Everything else is used by the body almost completely. To make this process even better, the softest part of the carcass is used. Regular consumption of this meat helps cope with fatigue. Beef is very useful for iron deficiency anemia. And those with high cholesterol are prescribed a diet with daily consumption of boiled red meat. In this case, in a few weeks the indicators decrease by 20%, which is an excellent result.
Basic recommendations
Beef is the meat of cattle that are specially fattened on farms for slaughter. Quality depends on a large number of factors: age and type of feed, content and sex of the animal. Even if we consider the carcass of one animal, the meat on it will not be the same. Those parts of the body where the strongest muscles are located will be the stiffest. Accordingly, when talking about how much protein is in beef, it must be understood that the body still has to extract and absorb it.
The most valuable are the dorsal and thoracic parts obtained from immature bulls and heifers. This high-quality meat is pink in color, has a pleasant smell and a delicate fibrous structure. But there should be practically no fat or films in it. These parameters do not affect how much protein is in beef, but they largely determine the degree of its absorption by the body.